Concepts
- Metals: Those elements which form positive ions by losing electrons all called metals. i.e. Copper , Iron , Aluminum, sodium etc.
- Physical Properties of Metals:
- Metallic lustra : In pure state, metals have a shining surface.
- Physical State: Generally solid at room temperature. Exception: Mercury is metal but in liquid form at room temp.
- Hardness: Most of the metals are hard. ie.e Iron , copper , tin etc.. Exception: some metals like lithium , sodium and potassium are so soft that they can be easily cut with a knife. They have low densities and low melting point.
- Density : Metals have high density Exception: sodium and potassium have low density.
- Ductility : A property of metal due to which a metal can be drawn into thin wires. Metals are generally ductile. Gold is the most ductile metal.
- Malleability : A property of metal due to which it can be beaten into thin sheets. Most of the metals are malleable. Gold and Silver are most malleable metals.
- Electrical Conductivity: Generally Metals are good conductors of electricity in solid state. Silver is the best conductor followed by copper , gold, aluminum and tungsten. Exception: Mercury is a bad conductor while lead is also most non-conducting.
- Thermal Conductivity : Generally Metals are good conductors of heat. ie. copper and silver. Exception: Mercury and lead are poor conductor of heat.
- Melting and boiling points: Generally Metals are have high melting & boiling points. Tungsten has the highest melting point among metals. Exception: Gallium and caesium have very low melting points. These two metals will melt even if we keep them on our plam.
- Sonority : The metals that produce a sound on striking hard surface are said to be sonorous. eg. school bells are made up of metals due to this property.
- Chemical Properties of Metals
- Metals reaction with air or oxygen to form metal oxide.
- For Example, Copper reacts with oxygen to form copper oxide.
- Metal + O2 → Metal oxide
- 2Cu + O2 → 2CuO ( Copper oxide ) ( black )
- 4Al + 3O2 → 2Al2O3 ( Aluminum Oxide )
- Some metal oxides such as aluminum oxides, zinc oxide show both acidic and basic behavior. Oxides of metals can react with both acids and bases to produce salt and water. Such oxides are known as Amphoteric Oxides.
- Metal Oxide+ Acid or Base → Salt + Water
- Al2O3 + 6HCl → 2AlCl3 + H2O

-
- Metallic oxides are insoluble in water but some of these dissolve in water to form hydroxides known as Alkalis.
- Na2O+ H2O → 2 NaOH ( Sodium Hydroxide ) ( Alkali )
- So Alkalis are the bases that dissolve in water and turns red litmus solution blue.
- Order of Reactivity of Metals with Oxygen : Na>Mg>Zn>Fe>Cu>Ag
- Metallic oxides are insoluble in water but some of these dissolve in water to form hydroxides known as Alkalis.
- Metals Reaction with water to form metal oxide and hydrogen gas. Since Metal oxides are solution in water so they dissolve in water further to form metal hydroxide.
- Metal+ Water → Metal oxide+ Hydrogen Gas
- Metal Oxide + Water → Metal Hydroxide
- Reactivity order of metals with water is : K>Na>Ca>Mg>Al>Fe>Pb>Cu>Ag>Au
- For Example
-
- 2Na + 2H2O → 2NaOH + 1H2
- 2Al + 3H2O → Al2O3 + 3H2
- 3Fe + 4H2O → Fe2O4 + 4H2
-
- Metals Reaction with dilute acids to form salt and hydrogen. For example, magnesium reacts with dilute hydrochloric acid to form magnesium chloride and hydrogen.
- Metal + Acid → Metal Salt + Hydrogen
- Mg + 2HCl → MgCl2 + H2
- Zn+ 2HCL → ZnCl2 + H2
- Fe+ 2HCL → FeCl2 + H2
- Reaction of Metals with Solutions of other Metal Salts
- Most Reactive Metals can displace less reactive metals from their compounds in solution or molten form. This type of reaction is called displacement reaction.
- Cu + 2AgNo ( Silver Nitrate ) → Cu( NO3)2 + 2Ag ( Silver )
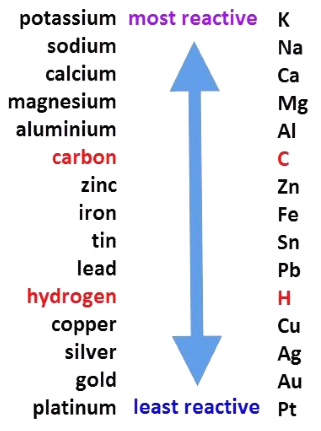
- Reactivity Series
The series in which metals are arranged in the decreasing order of reactivity is known as the Reactivity Series
- Non-Metals: Those elements which form negative ions by gaining electrons are called non-metals. ex. Iodine, Sulphur , oxygen, hydrogen. etc.
- Physical Properties of Non-Metals
- Lustre: no shining surface. do not have lustre. Exception: Diamond , graphite and iodine have lustre.
- Physical State: Either solids or gases. Exception: Bromine is liquid at room temperature.
- Softness: most of the non-metals are soft. Exception: only diamond is hardest known substance
- Malleability and ductility: Neither malleable nor ductile.
- Brittleness: Non-metals are brittle in nature.
- Electrical & Thermal Conductivity : Non-metals are poor conductors of heat and electricity. Exception: Graphite is a good conductor of electricity.
- Melting & Boiling Points : Generally non-metals have low melting & boiling points. But non-metals that are solids have comparatively. higher boiling points. i.e. : B , Si , C etc.
- Chemical Properties of Non-Metals:
- Non-metals react with oxygen to form non-metal oxide.
- Non-metal + Oxygen → Non-metal oxide
- C + O2 → CO2
- Non-metals do not react with water and acids to evolve hydrogen gas.
- Non-metals can react with salt solution; the more reactive element will displace the less reactive non-metal.
2 NaBr (aq) + Cl2(aq) → 2NaCl (aq) + Br2 (aq)Non-metals can also react with hydrogen to form hydrides.
H2(g) + S(l) → H2S(g)
- Non-metals react with oxygen to form non-metal oxide.
- Ionic Bond Formation
- Metals have a tendency to loose electrons to form cations ( +ve ions ) and non-metals have a tendency to gain electrons to form anions ( -ve ions )
- When metals and non-metals react each other, then both of them tries to achieve completely filled outermost shell by transfer of electrons. This type of chemical bond is called ionic bond.
- Ionic Compounds
Compounds formed due to the transfer of electrons from a metal to a non-metal are known as Ionic Compounds.

Properties of Ionic Compounds
- They are generally hard and solid.
- They have a high melting and boiling point.
- They are soluble in water but insoluble in inorganic solvents such as ether etc.
- They are conductors of electricity in molten and solution states.
Occurrence of Metals
Elements or compounds which occur naturally in earth crust are known as Minerals. Minerals from which pure metals can be extracted are known as Mineral Ores.

Extraction of pure metals from its ores/steps for extraction of metals from its ore
- The first step is the enrichment of the ore
- The second step includes extraction of metals
- Third steps involve refining of metal
Gangue - Ores contain different impurities in it such as sand, soil etc. These impurities are known as Gangue.
Extracting Metals which are low in activity series
Metals which are low in the activity series are unreactive. The oxides of such metals can be reduced to metals by heating alone. For Example, Cinnabar (HgS)

Extracting Metals in the middle of the Activity Series
These metals are moderately reactive. They exist as sulphides or carbonates in nature. Before reduction, metal sulphides and carbonates must be converted into metal oxides. Sulphide ores are converted into oxides by heating strongly in the presence of excess air, this is known as Roasting. Carbonate ores are converted into oxides by heating in limited air. This is known as Calcination.
Roasting
![]()
Calcination
![]()
Reduction-metal oxides can be reduced to metals using a reducing agent such as Carbon.
Extracting metals towards the top of the activity series
The metals are highly reactive. They cannot be obtained by heating. For Example, Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
At cathode Na+ + e- → Na
At anode 2Cl- → Cl2 + 2e-
Refining of Metals
Refining of impure metal is done using electrolytic refining. Impure copper is used as anode and a strip of pure copper is used as Cathode. Acidified copper sulphate is used as an electrolyte. When an electric current is passed through this, impure metal from the anode gets deposited in the electrolyte solution, whereas pure metal from the electrolyte is deposited at the cathode.
Deposition of insoluble residue formed from the dissolution of the anode during commercial electrolysis.

Fig.2. Electrolytic refining
Corrosion
It is the slow process of eating away of metals by the reaction of atmospheric air and moisture.
When exposed to moist air for a long period of time, metals become corroded. This is known as Corrosion. For Example, Silver reacts with moist air and becomes black in colour due to silver sulphide coating.
Iron + oxygen → Iron (III) oxide
Fe + O 2 → Fe2O3
Prevention of Corrosion
- Rusting of iron can be prevented by oiling, galvanising, painting, greasing etc.
- To protect steel and iron from rusting, a thin layer of zinc is coated on them, this is known as Galvanization.
Alloy
Mixture of two or more metals or metal and non-metal is known as Alloy. For Example,
- Brass is an alloy of copper and zinc.
- Bronze is an alloy of copper and tin.
- Solder is an alloy of lead and tin.

