Concepts
Acids
- Acid - A substance that produces hydrogen ions (H+) in aqueous solutions (water).For Example, Sulphuric Acid(H2SO4), Hydrochloric Acid (HCl)etc.
- They give a sour taste.
- Acids turn blue litmus to red. This is used as a confirmation test for the presence of acid.
- When acids react with metals, gases are evolved.
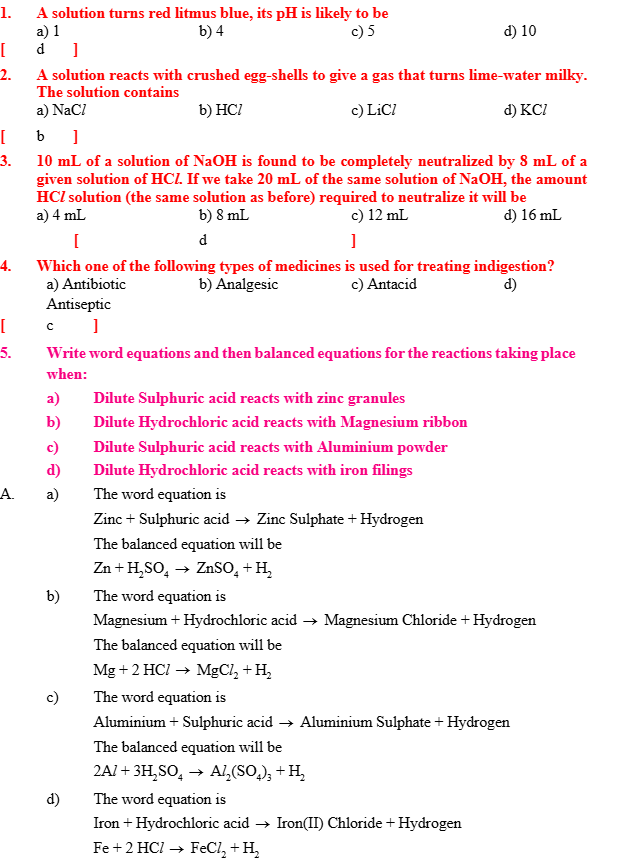
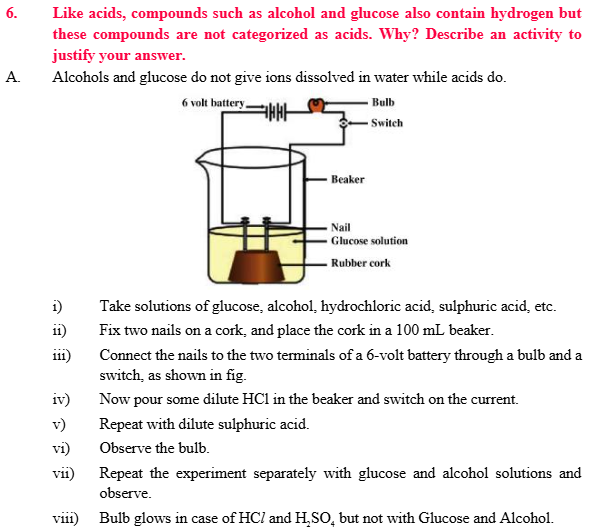
- They conduct electricity in solution form.
- They release H+ ions in aqueous solution.
Reactions with Acids
1. Reaction of Acid with Metal
- Acid + Metal → Salt + Hydrogen gas
- Mg + H2SO4 → H2 + Mg SO4
- Na (metal) + H2 SO4 (acid) → H2 (hydrogen gas) + Na SO4 (salt)
2. Reaction of Acid with Carbonates
- Na2 CO3 (s) + 2 HCl (aq) → 2NaCl (aq) + H2O(l) + CO2(g)
3. Reaction of Acid with Bicarbonates
- NaHCO3 (s) + HCl (aq) → NaCl(aq) + H2O (l) + CO2 (g)
Similarity between Acids and Bases
- Both acids and bases react with water. They produce ions in water
- Both acids and bases act as electrolytes, so are good conductors of electricity.
- Both of them change the colour of the litmus paper.
Classification of Acids
Acids are classified as Organic Acids and Mineral Acids. Acids that are derived from plants and animals are known as Organic Acids.
For Example, Citric Acid from fruit. Mineral acids are inorganic acids such as Sulphuric Acid. They are dangerous to be used, so need more precautions.
Acids are also classified as Strong Acids or Weak Acids. A strong acid is an acid that completely dissociates into ions in aqueous solutions. For Example, Sulphuric Acid, Hydrochloric Acid.
![]()
A weak acid is one that does not dissociate completely into ions in aqueous solutions. For Example, Acetic Acid.
![]()
Acids can also be as Dilute Acid and Concentrated Acids. The one which has a low concentration of acids in an aqueous solution are known as Dilute Acids whereas the one which has a high concentration of acids in an aqueous solution are known as Concentrated Acids.
It is advisable to add acid to water and not vice versa because a large amount of heat is released if water is added to acid. This released heat is large enough to cause harm.
Acids can also be classified based on the number of hydrogen ions. Monoprotic acid is the one that gives one mole of hydrogen ions per mole of acid, such as HCl. Diprotic Acid is one that produces two moles of hydrogen ions per mole of acid. For Example, H2SO4.
Bases
- Base - A substance that produces hydroxide ions (OH–) in aqueous solutions. For Example - Sodium Hydroxide (NaOH), Potassium hydroxide (KOH)
- Bases that are water-soluble are known as Alkalis.
- They turn red litmus to blue.
- They have a bitter taste.
- They also produced carbon dioxide when reacted with carbonates.
- They also evolved hydrogen gas when bases react with metals.
Reactions of Bases
1. Reaction with Metals
- Base reacts with metals and produces hydrogen gas.
- 2NaOH + Zn → Na2 → Na2ZnO2 + H2
2. Reaction with Acids
- Base reacts with acids to form salts. For Example,
- KOH + HCl → KCl + H2O
3. Reaction with Non-metallic Oxides
- Base reacts with non-metallic oxides to form salt and water.
- 2NaOH + CO2 → Na2CO3 + H2O
|
Strong Acids
An acid which completely dissociates into its ions in aqueous solution. For example: Hydrochloric acid (HCl), Sulphuric acid (H2SO4), Nitric acid (HNO3) |
Strong Base
A base which completely dissociates into its ions in aqueous solution. For example: Sodium hydroxide (NaOH), Potassium hydroxide (KOH) |
|
Weak Acids
An acid which does not completely dissociate into its ions in aqueous solutions. For example: Acetic acid (CH3COOH), Carbonic acid (H2CO3) |
Weak Base
A base which does not completely dissociate into its ions in aqueous solution.
For example: Ammonium hydroxide (NH4OH).
|
Classification of Bases
Bases are classified as Strong Base and Weak Base. A strong base is one that dissociates completely into its ions in an aqueous solution. For Example, NaOH.
A weak base is one that does not dissociate completely into its ions in aqueous solutions. For Example, Ammonium Hydroxide, NH4OH
Bases are also classified as Dilute Base and Concentrated Base. The solution which has a low concentration of base in an aqueous solution is defined as Dilute Base whereas the one which has a high concentration of base in an aqueous solution is known as a Concentrated Base.
Strength of Acid or Base Solutions
The dissociation constant of a weak acid or weak base can be represented as-

Suppose HA is a weak acid, then dissociation constant is represented as-

Concept of pH scale
Strength of an acid or base can be determined using a pH scale. It is a scale to measure the hydrogen ion concentration in a solution. The p stands for ‘potenz’, it is a German word which means power.
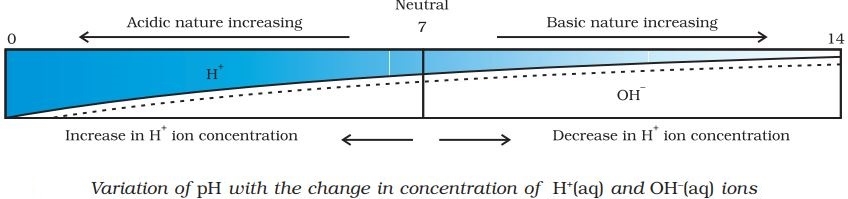
For water or neutral solutions : pH = 7
For acidic solutions : pH < 7
For basic solution : pH > 7
Importance of pH in everyday life
(i) pH in our digestive system: Our stomach produces hydrochloric acid that helps in the digestion of food. During indigestion, the stomach produces too much acid and this causes pain and irritation. To get rid of this pain, antacids like magnesium hydroxide [Mg(OH)2] also known as milk of magnesia and sodium hydrogen carbonate (baking soda) are used to neutralize excess acid.
(ii) Tooth decay caused by acids: Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating. When the pH of acid formed in the mouth falls below 5.5, tooth-decaying starts. The best way to prevent this is to clean the mouth after eating food. Using toothpastes, which are generally basic, for cleaning the teeth can neutralise the excess acid and prevent tooth decay.
- If pH is equal to 7, it means the solution is neutral.
- If pH is greater than 7 means alkaline solution.
- If pH is less than 7 means the solution is acidic.

Fig.1. pH scale
Importance of pH
- Human body works at a pH of about 7.4.
- The stomach has a pH of about 2 due to the presence of hydrochloric acid in it. It is needed for the activation of pepsin protein required for protein digestion.
- When we eat food containing sugar, then the bacteria present in our mouth break down the sugar to form acids. This acid lowers the pH in the mouth. Tooth decay starts when the pH of acid formed in the mouth falls below 5.5. This is because then the acid becomes strong enough to attack the enamel of our teeth and corrode it. This sets in tooth decay. The best way to prevent tooth decay is to clean the mouth thoroughly after eating food.
- Many animals and plants protect themselves from enemies by injecting painful and irritating acids and bases into their skin.
- When a honey bee stings a person, it injects an acidic liquid into the skin. Rubbing with a mild base like baking soda solution on the stung area of the skin gives relief.
- When a wasp stings, it injects an alkaline liquid into the skin. Then rubbing with a mild acid like vinegar on the stung area of the skin gives relief.
- Soil pH and plant growth: Most plants grow best when the pH of the soil is close to 7. If the soil is too acidic or basic, the plants grow badly or do not grow at all. The soil pH is also affected by the use of chemical fertilisers in the field. Chemicals can be added to soil to adjust its pH and make it suitable for growing plants. If the soil is too acidic then it is treated with materials like quicklime or slaked lime. If the soil is too alkaline then alkalinity can be reduced by adding decaying organic matter.
Salts
When acid and base neutralise, salts are formed. Strong acid and strong base combine to form neutral salt.
NaOH + HCl → NaCl + H2O
Eq.1. Formation of Neutral Salt
Strong acid and weak base combine to form acidic salt. For Example, Hydrochloric Acid and ammonium hydroxide combine to form ammonium chloride. Other examples, are sodium hydrogen carbonate, sodium hydrogen sulphate etc.
HCl + NH4OH → NH4Cl + H2O
Eq.2. Formation of Acidic Salt
Similarly, weak acid and strong base combine to form basic salt. For Example, Acetic Acid and sodium hydroxide combine to form sodium acetate. Other examples are calcium carbonate, potassium cyanide etc.
CH3COOH + NaOH → CH3COONa + H2O
Eq.3. Formation of Basic Salt
The most common salt is table salt or sodium chloride (NaCl).
Indicators
They are the substances that indicate the acidic or basic nature of the solution using colour change. For Example, litmus solution, methyl orange, phenolphthalein, methyl red etc. Acids convert blue litmus paper red in colour. Bases turn red litmus blue. Phenolphthalein remains colourless in presence of acids but turns pink in presence of bases.
Some Important Chemical Compounds and their uses
| Chemical Compounds | Preparation | Uses |
| Common Salt (NaCl)
(Sodium Chloride) |
1. NaOH + HCl → NaCl + H2O
2. From seawater by evaporation 3. From underground deposit {Large crystals of common salt are found in underground deposits which are brown due to the presence of impurities in them. It is mined from underground deposits like coal.} |
1. Raw material for making large numbers of useful chemicals in industry. Eg: NaOH (caustic soda), Na2CO3 (washing soda), NaHCO3 (baking soda).
2. Preservative in a pickle and curing meat and fish. 3. To melt ice and clear roads in winters in cold countries. 4. Used in the manufacturing of soap. |
| Caustic Soda (NaOH)
(Sodium Hydroxide) |
Passing electricity through a concentrated solution of NaCl (called 'brine')
2NaCl (Brine) + 2H2O2NaOH (Caustic Soda) + Cl2 + H2 At anode (+ve electrode): Cl2 is produced At the cathode (-ve electrode): H2 is produced It is called a chloro-alkali process because products formed are chlorine (Chloro) and NaOH (alkali). |
Uses of H2
1. Hydrogenation of oil to get vegetable ghee (margarine) 2. To make ammonia for fertilisers 3. In fuel for rockets. Uses of Cl2 1. In water treatment 2. To clean water in swimming pools 3. To make plastic, e.g. PVC 4. To make CFCs, chloroform, dyes etc. Uses of NaOH 1. Used in making soap and detergent. 2. Used in manufacturing of paper 3. Degreasing metals 4. Refining oil 5. Making dyes and bleaches Uses of HCl 1. Cleaning steel 2. Preparation of chloride, e.g. NH4Cl 3. In making medicines and cosmetics 4. In making plastics, PVC etc. |
| Baking Soda (NaHCO3)
(Sodium Hydrogencarbonate) |
NaCl + NH3 + H2O + CO2 → NaHCO3 + NH4Cl
Properties Action of Heat:
|
1. Used as an antacid in medicine to remove the acidity of the stomach
2. Used in making baking powder (Basic soda + tartaric acid) NaHCO3 + H⊕ (from mild acid) → Na⊕ (sodium salt of acid) + CO2 + H2O The CO2 produced during the process gets trapped in wet dough and bubbles out slowly to make the cake 'rise' so that it becomes soft and spongy. Tartaric acid neutralises it, and so it has a pleasant taste. 3. Used in soda-acid fire extinguisher |
| Washing Soda (Na2CO3.10H2O)
(Sodium Carbonate) |
Na2CO3 + 10 H2O → Na2CO3.10H2O
Preparation of Na2CO3 {NaCl + NH3 + H2O + CO2 NaHCO3 + NH4Cl NaHCO3 → Na2CO3 + CO2 + H2O} |
1. Used in glass, soap and paper industries
2. Used in manufacturing of sodium compounds such as Borax 3. Cleaning agent for domestic purpose 4. Remove permanent hardness of water |
| Bleaching Powder (CaOCl2)
Calcium Oxychloride |
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Slaked Lime Calcium Oxychloride Properties CaOCl2 + H2SO4 → CaSO4 + Cl2 + H2O The Cl2 produced by the action of dilute acid acts as a bleaching agent. |
1. For bleaching cotton and linen in the textile industry, for bleaching wood pulp in paper factories, for bleaching washed clothes in laundry
2. The oxidising agent in chemical industries 3. Disinfecting drinking water |
| Plaster of Paris (P.O.P) (CaSO4.1/2 H2O)
(Calcium Sulphate Hemihydrate) |
CaSO4.H2O (Plaster of Paris) +3/2 H2O * Heating of gypsum should not be done above 100oC as above that temperature, the water of crystallisation will be eliminated and anhydrous CaSO4 will be obtained. This anhydrous CaSO4 is known as Dead Burnt Plaster. * CaSO4.1/2 H2O means that two molecules of CaSO4 share one molecule of water. Properties POP has a remarkable property of setting into a hard mass on wetting with water, as gypsum is formed. CaSO4.1/2 H2O (P.O.P) + 1/2 H2O → CaSO4.2H2O (Gypsum set as hard mass) Hence, P.O.P should be stored in moisture-proof containers as moisture can cause the slow setting of P.O.P by hydrating it. |
1. Used in hospital for setting fractured bones in the right position to ensure correct healing.
2. Making toys, decorative materials, cheap ornaments, and casts of statues. 3. Used as fire-proofing material 4. Used in chemistry labs for setting air gaps in apparatus. 5. Making smooth surfaces, such as For making ornamental designs on ceilings of houses and other buildings |
NCERT Questions & Answers