Concepts
Understand Fundamentals
- Anloine Lavoiser - Father of modern chemistry classified all elements into metals , nonmetals and metalloids.
- Metals: Most metals are hard except Na & K , shiny , malleable , ductile , sonorous , good conductors . Ex: Iron , Copper , Gold , Silver , Aluminium , Zinc , Lead etc. Mercury is the only metal which is liquid.
- Non-Metals : Mostly soft , shiny/dull , brittle , neither ductile nor malleable , nor sonorous ( do not produce sound ) , non conductor except ( graphite )
- Metalloids: Show the properties of metals as well as nonmetals. Ex.Boron , Silicon , Arsenic , Germanium
- Chemical Properties of Metals
- Reaction with oxygen : Metal + Oxygen ⇨ Metal oxide
- 4Na + O2 ⇨ 2Na2O
- 4K + O2 ⇨ 2K2O
- 2Mg + O2 ⇨ 2MgO
- 2Zn + O2 ⇨ 2ZnO
- 3Fe + 2O2 ⇨ Fe3O4
- 2Cu + O2 ⇨ 2CuO
- 4Al + 3O2 ⇨ 2Al2O3
- Lithium, potassium, sodium, etc. are known as alkali metals. Alkali metals react vigorously with oxygen.
- Iron fillings give sparkle in flame when burnt.
- Reaction of Metals With Water : Metal + Water ⇨ Metal hydroxide + Hydrogen
- K + H2O ⇨ KOH + H2
- Na + H2O ⇨ NaOH + H2
- Ca + 2H2O ⇨ Ca(OH)2 + H2
- Mg + 2H2O ⇨ Mg(OH)2 + H2
- 2Al + 3H2O ⇨ Al2O3 + 2H2
- Zn + H2O ⇨ ZnO + H2
- 3Fe + 4H2O ⇨ Fe3O4 + 4H2
- Reaction of water with sodium and potassium is highly exothermic, and reaction mixture catches fire.
- Reaction between water and calcium is exothermic, but reaction mixture does not catch fire.
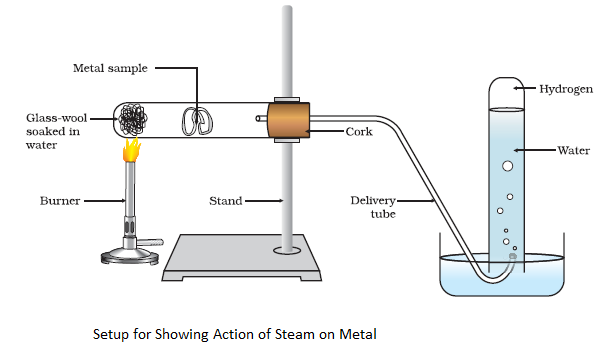
- Magnesium does not react with cold water but reacts with hot water.
- Aluminium, iron and zinc do not react with cold or hot water but they react with steam.
- Some metals do not react with water at all, e.g. lead, copper, silver and gold.
- Reaction with Acid : Metal + dil. acid ⇨ Metal salt + Hydrogen
- 2Na + 2HCl ⇨ 2NaCl + H2
- 2K + H2SO4 ⇨ K2SO4 + H2
- Mg + 2HCl ⇨ MgCl2 + H2
- 2Al + 6HCl ⇨ 2AlCl3 + 3H2
- Zn + H2SO4 ⇨ ZnSO4 + H2
- Copper, gold and silver are known as noble metals. These metals do not react with dilute acids.
- Reaction with Bases/Alkalies:
- When alkali (base) reacts with metal, it produces salt and hydrogen gas. Sodium hydroxide gives hydrogen gas and sodium zincate when reacts with zinc metal. Sodium aluminate and hydrogen gas are formed when sodium hydroxide reacts with aluminium metal.
- 2NaOH+Zn⟶Na2ZnO2+H2
- 2NaOH+2Al+2H2O⟶2NaAlO2+2H2
- Metal Displacement Reactions:
- Mg + CuSO4 ⇨ MgSO4 + Cu
- Zn + CuSO4 ⇨ ZnSO4 + Cu
- Fe + CuSO4 ⇨ FeSO4 + Cu
- Reactivity of some metals are given in descending order
K > Na > Ca > Mg > Al > Zn > Fe > Pb > Cu
- Reaction with oxygen : Metal + Oxygen ⇨ Metal oxide
- Chemical Properties of Nonmetals
- Reaction of non-metals with oxygen: Non-metal + Oxygen ⇨ Non-metal oxide
- C + O2 ⇨ CO2 + Heat
- 2C + O2 ⇨ 2CO + Heat
- S + O2 ⇨ SO2
- 2H2 + O2 ⇨ 2H2O
- When carbon dioxide is dissolved in water, it gives carbonic acid.CO2 + H2O ⇨ H2CO3
- When sulphur dioxide is dissolved in water, it gives sulphurous acid.
SO2 + H2O ⇨ H2SO3
- When sulphur dioxide reacts with oxygen, it gives sulphur trioxide
2SO2 + O2 ⇨ 2SO3
- When sulphur trioxide is dissolved in water, it gives sulphuric acid.
SO3 + H2O ⇨ H2SO4
-
Reaction of non-metal with chlorine
When a non-metal reacts with chlorine, then chloride is formed. General equation for this reaction is given below.
Non-metal + Chlorine ⇨ Non-metal chloride
When hydrogen reacts with chlorine, then hydrogen chloride is formed. Following is equation for this reaction.
H2 + Cl2 ⇨ 2HCl
When phosphous reacts with chlorine, then phosphorus trichloride is formed. Following is equation for this reaction.
P4 + 6Cl2 ⇨ 4PCl3
- Reaction of non-metals with oxygen: Non-metal + Oxygen ⇨ Non-metal oxide
Mock Test
Test your knowledge

